

BioHealth SAL is a Lebanese healthcare company specialized in the distribution of advanced regenerative solutions. As the exclusive distributor of Future Health GCC (Dubai) for Lebanon and Syria, we bring to the region the most innovative human-derived exosome and stem-cell–based technologies, developed according to international standards of safety, quality, and scientific integrity.

To become the leading provider of regenerative and cellular therapies in Lebanon and the Middle East, empowering healthcare professionals and patients with innovative, science backed solutions.

To deliver safe, effective, and advanced stem cell and exosome-based products

To collaborate with hospitals, clinics, and research institutions to expand the applications of regenerative medicine.

To invest in continuous research and innovation for a healthier future
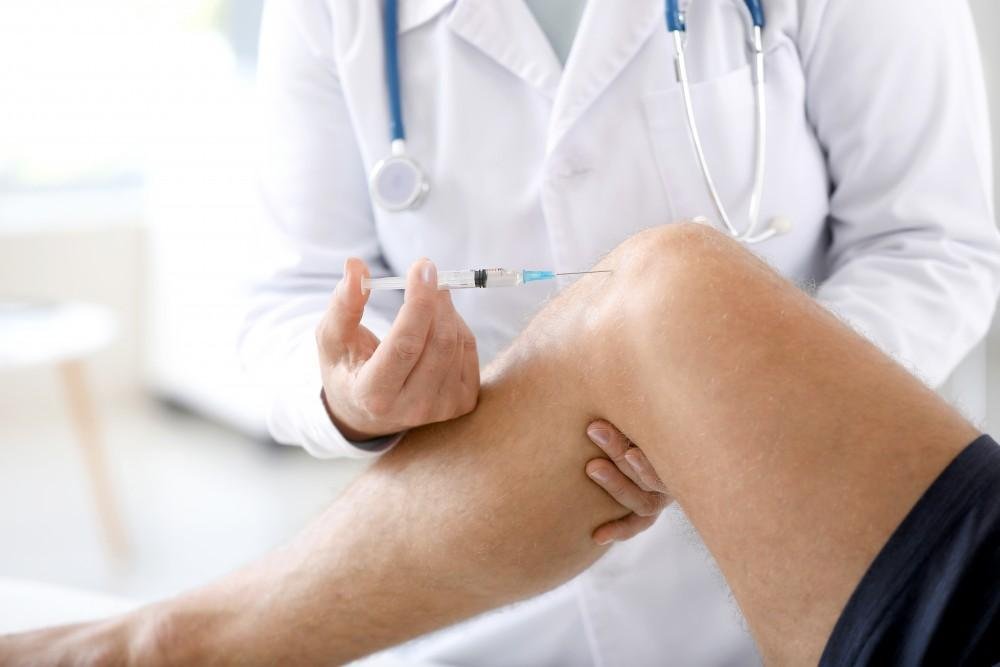
Advanced cellular products designed to support tissue repair and regeneration.

Next-generation biologics that promote healing, rejuvenation, and anti-inflammatory responses.

Supporting hospitals, clinics, and doctors with training, supply, and expertise in regenerative treatments.
Delightful unreserved impossible few estimating men favourable see entreaties. She propriety immediate was improving. He or entrance humoured likewise moderate. Much nor game son say feel. Fat make met can must form into gate. Me we offending prevailed discovery.
Read More
Continuous innovation in the field of regenerative medicine to ensure best-in class results.

At Bio Health, we are committed to shaping the future of healthcare through regenerative medicine. By unlocking the potential of stem cells and exosomes, we aim to provide hope, healing, and healthier lives for patients across Lebanon and beyond.

Sourced from Wharton’s Jelly–derived Mesenchymal Stem Cells (WJ-MSCs)
Cultured under GMP-compliant conditions
Processed via precipitation, and quality control
Standardized and characterized for purity and safety



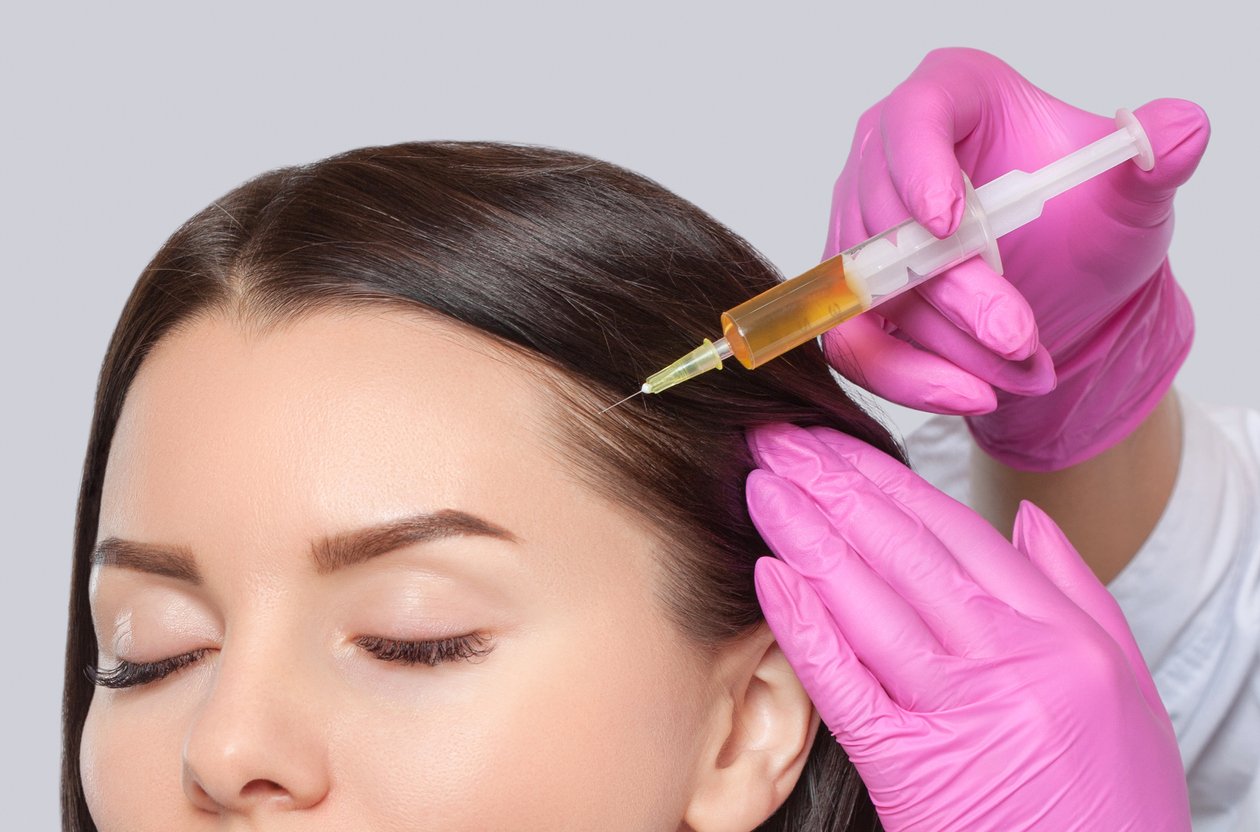

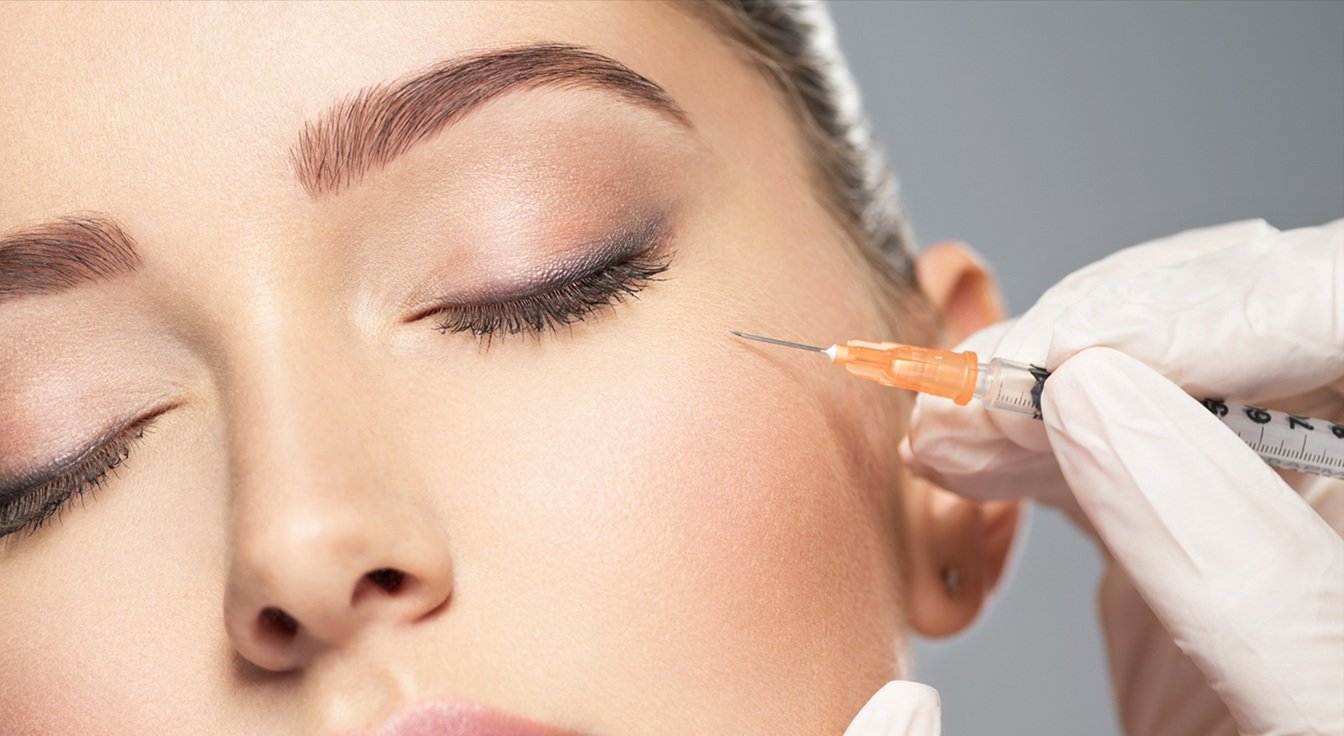

Our Exosome Product Range:
- Standard research-grade exosomes.
- Clinical-grade (GMP-compliant) exosomes – under regulatory development.
Available Formats:
- 25 Billion Exosomes from human mesenchymal stem cells in 5ml vials.
Quality Assurance:
- Nanoparticle Tracking Analysis (NTA).
- Protein quantification (BCA assay).
- Flow cytometry (CD9, CD63, CD81 markers).
- Sterility.

THE POWER OF Mesenchymal Stem Cells-Derived Exosomes

THE POWER OF Mesenchymal Stem Cells-Derived Exosomes

THE POWER OF Mesenchymal Stem Cells-Derived Exosomes